
Helium – Properties Element Helium Atomic Number 2 Symbol He Element Category Noble Gas Phase at STP Gas Atomic Mass 4.0026 Density at STP 0.1785 Electron Configuration 1s2 Possible Oxidation States 0 Electron Affinity - Electronegativity - 1st Ionization Energy 24.5874 Year of Discovery 1895 Discoverer Ramsey, Sir William & Cleve, Per Teodor Thermal properties Melting Point -272.2 Boiling Point -268.9 Thermal Conductivity 0.1513 Specific Heat 5.193 Heat of Fusion - Heat of Vaporization 0.0845 Its boiling point is the lowest among all the elements. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas, the first in the noble gas group in the periodic table. In general, helium will not combine with itself to create molecules, but is found as a single atom.Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure.It is used instead of nitrogen because it will not dissolve in the blood and will leave the human body quicker. It is often mixed with oxygen in scuba air tanks to dilute the oxygen.Helium has never been observed by scientists to bond with another element to form a compound.This causes people's voices to get high pitched and squeaky when they breathe helium (note: never breathe helium as you can suffocate if you breathe too much). The speed of sound in helium is around three times the speed of sound in the air.The most abundant of the helium isotopes is Helium-4 which was largely created at the beginning of the universe. There are eight known isotopes of helium. Helios is also the name of the Greek god of the Sun. Helium gets its name from the Greek word "helios" meaning "sun". The element wasn't found on Earth until 1895. He noticed the new element when studying a solar eclipse. Helium was first discovered in 1868 by astronomer Pierre Janssen. Other applications include silicon wafers for electronics and as a protective gas for arc welding. The largest industrial user of helium gas is MRI scanners which use the gas to keep the superconducting magnets cool. It is not as light at hydrogen, but is a much safer gas as hydrogen is very flammable. Helium is used in balloons and airships to make them float. This conversion is called nuclear fusion. This creates the energy, heat, and light that powers the stars and the sun. Deep inside a star, intense pressures cause hydrogen atoms to convert into helium atoms. Helium is constantly being produced at the internal cores of stars. Helium from radioactive decay can be found trapped underground in natural gas reservoirs. However, new helium is created in the center of stars and also as part of radioactive decay on Earth. Scientists believe that most of the helium in the universe was created at the formation of the universe. There is very little in the Earth's atmosphere because it is so light that it eventually escapes into outer space. This makes it very unreactive and non-flammable. This means that its outside electron shell is filled with electrons. Helium is one of the inert or noble gases. Helium is the only element that does not solidify under ordinary pressures and remains a liquid even at absolute zero.
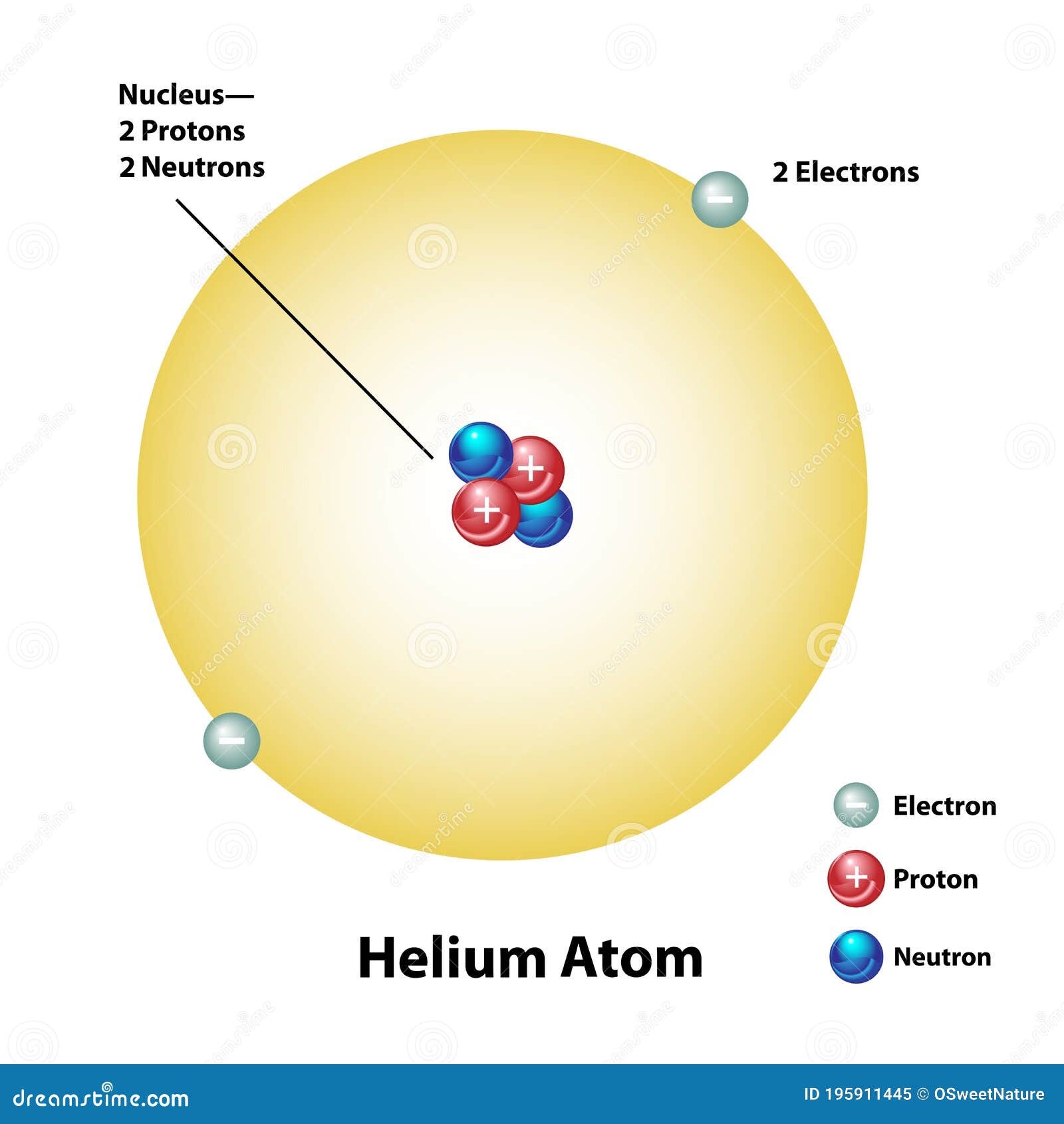
It has very low boiling and melting points, meaning that it is generally found in the gas phase except under the most extreme of conditions. It is at the top of the noble gas group in the periodic table.Īt room temperature helium is an odorless, tasteless, colorless gas. Helium is the second lightest and second most common element in the universe. Classification: A noble gas and a nonmetal.


 0 kommentar(er)
0 kommentar(er)
